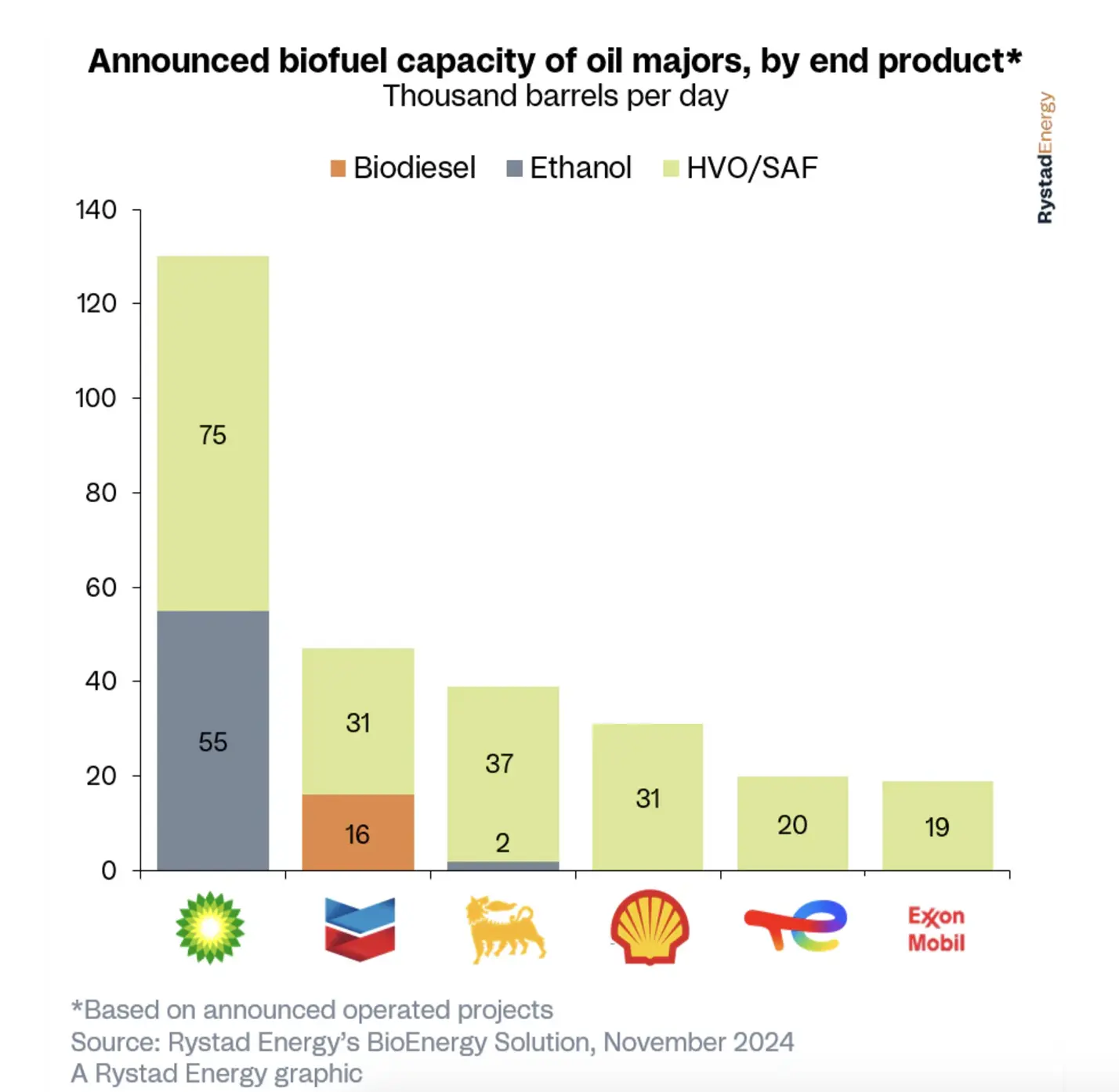
Researchers have come up with an interesting new way to produce H2 in marine vehicles
A team of MIT researchers have discovered a fresh take on H2 production for a hydrogen reactor design that could be used to power marine vehicles such as submarines, which already draw in seawater.
The fuel can be produced using only seawater, soda cans and coffee grounds
The researchers focused on this chemical reaction because it could offer marine vehicles a new source of power in a world that is working to decarbonize its energy production. The hydrogen reactor would use this energy dense fuel for carbon emission free operation with water as its only byproduct.
That said, one of the top challenges for its use in areas such as marine transportation is that H2 as a fuel is hard to store and transport when compared to more conventional fossil fuels. It can easily leak from containers and piping due to its exceptionally small molecular size. Not only is this loss expensive over time, but it can also be problematic to the atmosphere when too much H2 is added at once.
MIT’s hydrogen reactor is part of a look toward future systems
The new design would produce the H2 on demand right on the marine vehicle. Then, it could be used in the hydrogen reactor without having to store massive amounts of fuel that is difficult to contain. Instead, this system would require only aluminum pellets, which are notably easier to work with and are considerably more stable.
The researchers tested the reaction using a single 0.3 g aluminum pellet in fresh, deionized water. Within five minutes, the process produced 400 ml of H2. Using a scaled-up method, the researchers estimated that by using one gram of aluminum pellets, the result would be 1.3 L of hydrogen within the same amount of time.
The method is based on a relatively straightforward chemical reaction as the reaction between aluminum and oxygen is quite powerful. Therefore, when the aluminum is submerged into water, it rapidly draws the O off the H2O, leaving the H2 to bubble out of the liquid.
The challenge
Still, even with this simple and inexpensive method, there has yet to be a perfect solution for H2 production. In this case, a primary challenge is that the reaction is short. As it takes place, a thin aluminum oxide layer creates a buildup on the surface of the metal. This creates a barrier between the water and the aluminum beneath. As a result, the potential for a reaction between them is reduced and then eventually blocked.
Prior study has indicated that bringing in other metals such as gallium can prevent that buildup from occurring by breaking down the layer of aluminum oxide as it forms. Therefore, the researchers pretreated the aluminum pellets they used in the test using a gallium and indium allow. This made it possible to keep the hydrogen reactor running longer.
Rising price of the hydrogen reactor materials
 That said, this brings in a challenge that is experienced throughout the majority of H2 production methods, which is that it involves using rare and/or expensive metals. Gallium and indium are both rare and expensive.
That said, this brings in a challenge that is experienced throughout the majority of H2 production methods, which is that it involves using rare and/or expensive metals. Gallium and indium are both rare and expensive.
The researchers worked to overcome that challenge through additional tests, in which they discovered that when the reaction took place within an ionic solution, the expensive alloy would clump up so that it could be collected for reuse.
Seawater, as it happens, is an ionic solution. While seawater did slow the reaction down to two hours instead of five minutes, when the team tossed old coffee grounds into the reactor, the reaction speed returned to only five minutes once more due to the presence of imidazole, a part of the caffeine compound.