This story was produced in partnership with Atlanta News First.
The bruises on Alexandria Pittman’s body wouldn’t go away. Nor would the aches that plagued her at her new job at a distribution center in Lithia Springs, a small town 17 miles west of Atlanta, sorting and repackaging boxes containing medical devices. She was convinced the symptoms were connected to the job.
Pittman had applied to the position at the warehouse, run by the medical supply company ConMed, after learning about the opening from her fiancé, Derek Mitchell, who delivered products there. Every day she’d come home and complain to him about the mysterious aches and marks. At first, Mitchell tried to reassure her, guessing that the bruises were probably from bumping up against something. “I really didn’t think nothing of it,” he recalled.
Then, in the spring of 2019, came a surprising revelation. ConMed managers announced that the seemingly innocuous products in the boxes they were packaging had been sterilized with ethylene oxide, which the U.S. Environmental Protection Agency considers a carcinogen and is linked to lung and breast cancers as well as diseases of the nervous system. Suddenly, Pittman began connecting the dots between her symptoms and those of her colleagues. It would later emerge that at least 50 warehouse workers experienced a slew of health effects tied to ethylene oxide exposure, including seizures, vomiting, and trouble breathing. Ambulances were routinely called to the facility after workers collapsed, convulsed from seizures, or broke out in hives. Several — including Pittman — developed cancer.
Since ConMed came clean about the workers’ exposure to ethylene oxide, Pittman has suffered four strokes and had brain surgery. She’s currently undergoing chemotherapy for myeloma, according to multiple claims she has filed with the Georgia State Board of Workers’ Compensation for help paying her medical bills. After the second stroke, Mitchell was unable to care for her, and she moved in with her mother where she now lives. Mitchell and Pittman had planned to marry, but the $5,000 ring Mitchell purchased now sits collecting dust.
“It just corrupted everything that she ever wanted to do in life,” said Mitchell. “She can’t talk, and she’s being fed through a tube.”
The ethylene oxide that Pittman and dozens of her coworkers were exposed to wasn’t supposed to have made it to the warehouse at all. At a sterilization plant 12 miles down the road, the chemical had been used to fumigate products before they were sent to the warehouse, a standard procedure for making sure that medical equipment is antiseptic and safe to use in hospitals across the country. More than 50 percent of all U.S. medical supplies are sterilized by ethylene oxide, due to the chemical’s unique ability to penetrate porous surfaces without causing damage.
Ethylene Oxide Facts
But over the past few years — beginning with findings by the Occupational Safety and Health Administration, or OSHA, in 2019 and Georgia’s Environmental Protection Division, or EPD, in 2020 — regulators have learned that some amount of ethylene oxide travels out of sterilization facilities on the treated products. In the hours and weeks following application, the chemical evaporates, or off-gasses, turning the buildings where these products are stored into potentially significant sources of toxic air pollution — particularly for workers like Pittman who handled the boxes directly.
The dangers came as a surprise to warehouse workers and regulators alike. Georgia EPD officials had originally only set out to monitor ethylene oxide levels around the industrial sterilization facilities fumigating medical equipment. The EPA had just published modeling that suggested high levels of cancer risk around the country’s medical sterilization facilities, and Georgia regulators wanted to assess the plants in their jurisdiction. (The modeling incorporated the results of a 2016 study that found ethylene oxide to be 30 times more toxic to adults and 60 times more toxic to children than previously known.)
After finding elevated levels of ethylene oxide outside of a Becton Dickinson sterilization plant in Covington, a city southeast of Atlanta, officials asked a state judge to temporarily shut the operation down while further testing took place. As part of a consent decree reached in October 2019, the company would not only have to install new technology at its sterilization plants to reduce its emissions, but also test the air coming from its warehouse to ensure that emissions were below the legal limit there as well. The results of this testing showed that the warehouse was emitting nearly 5,600 pounds of ethylene oxide per year — about nine times as much as the sterilization facility when it was still operational, and higher than almost a third of all sterilizer plants in the country. (Georgia requires an industrial facility emitting more than 4,000 pounds of a hazardous air pollutant per year to obtain a permit from the state allowing it to do so.)
“We basically cited the facility for failure to have an air permit, and we required them to control their air emissions,” recalled Jim Boylan, head of the air protection branch at the Georgia Environmental Protection Division, in an interview. “That’s how it all started.”
When he realized that Becton Dickinson couldn’t be the only company storing medical products recently sterilized with ethylene oxide, Boylan assigned several engineers in the EPD’s inspection program to search for similar operations. They had their work cut out for them — because the risk of exposure at these warehouses is such a new concern, there is no comprehensive public or government data on the identity, location, or number of these facilities in Georgia or any other state, let alone any kind of risk assessments. Inspectors scoured the internet and made in-person visits to warehouses to identify potential sources of emissions. Their research revealed that in some cases, warehouse operators were aware that the medical devices they stored were releasing a carcinogen that could be poisoning their workers.
All told, interagency emails obtained by Grist through a Freedom of Information Act request show that inspectors initially identified seven warehouses in Georgia storing products that had been sterilized with ethylene oxide; four emitted enough of the chemical to require air permits and the installation of emission-reduction equipment. Those facilities are on track to receive their permits in the coming months. While these measures may protect residents who live near the warehouses, they don’t guarantee the protection of the workers who may be experiencing exposure day in and day out. The responsibility of safeguarding workers falls to OSHA, which has not investigated ethylene oxide levels at three of the four warehouses that Georgia regulators have identified as requiring permits.

Court documents include photos of a ConMed warehouse allegedly containing rows of packages of medical devices sterilized with ethylene oxide.
PeachCourt

According to court documents, cardboard and wood absorb ethylene oxide. The more dense the material, the slower it is to evaporate.
PeachCourt
Frances Alonzo, a spokesperson for the federal Department of Labor, said that OSHA evaluates employers “on a case-by-case basis based on OSHA standards, employer records, interviews, observations on a walk-through, measurements, and air sampling.” (In a follow-up email, Alonzo told Grist that OSHA’s ethylene oxide standards were established in 1984 and that the agency does not have plans to update it.) The agency focuses its resources on workplaces where employees are in imminent danger and conducts follow-up investigations to ensure any previously identified violations have been addressed.
In the ConMed case, after an initial investigation in 2019 identified a slew of violations, OSHA conducted two follow-up investigations. Those inspections revealed “no violations of OSHA standards or serious hazards,” Alonzo said. In 2020, Pittman and dozens of her coworkers filed a lawsuit against ConMed and the sterilization company that shipped products to the warehouse, but the claims were later dismissed by the judge.
Despite all the work still to be done by regulators, Georgia is relatively far ahead of the curve on addressing ethylene oxide emissions from warehouses. Most other states have yet to examine whether warehouses in their jurisdiction are storing sterilized products — and if emissions from the facilities put workers and nearby residents at risk. A review of public records submitted to the EPA and state regulators revealed that there are dozens of such warehouses across the country, suggesting there are thousands of workers like Pittman, unknowingly and routinely exposed to ethylene oxide. These nondescript facilities are hiding in plain sight in places as disparate as Quincy, Massachusetts; Richmond, Virginia; and Tempe, Arizona.
Warehouses that store products sterilized with ethylene oxide pose “a deep threat to communities, and unfortunately, because we don’t really know or have as much information as we should about where those warehouses are, it’s an unknown source of major ethylene oxide emissions,” said Marvin Brown, an attorney with the environmental nonprofit Earthjustice.
Grist contacted the environmental agencies of 10 states that are home to multiple medical supply sterilization facilities. Most agencies said that they did not regulate warehouses that stored products sterilized with ethylene oxide and pointed to federal regulations that require them to oversee sterilization and manufacturing facilities — but not warehouses or distribution centers. Apart from Georgia, the South Coast Air Quality Management District, a regulator that serves a portion of Southern California, is a lone outlier. The agency is currently in the process of finalizing a rule requiring warehouses that store ethylene oxide products to conduct air quality monitoring.
The federal government, for its part, hasn’t yet addressed major loopholes that exempt warehouses from emissions rules. Because ethylene oxide is toxic in such small amounts, officials have had trouble regulating emissions from the sterilization facilities themselves, in many cases permitting emissions that they later discover generate levels of cancer risk that exceed federal public health standards.
While the EPA introduced regulations to reduce ethylene oxide emissions from sterilization facilities last year, its rules only apply to warehouses when they are located on the same property as sterilization facilities. But not only do many companies store their products at warehouses tens or even hundreds of miles away, they also often contract third-party logistics providers to do the job for them. That means products may be warehoused at facilities owned by subsidiaries or entirely separate logistics firms. Some companies have reported using FedEx facilities to store sterilized products. To make matters more complicated, sterilizers have largely been unwilling to disclose the locations of their storage facilities, citing those details as “confidential business information” not subject to public disclosure, according to records submitted to the EPA.

Environmental advocates and public health experts interviewed for this story worried that these informational gaps as well as the findings in Georgia could indicate a substantial and invisible public health threat affecting communities across the country — and one that the EPA should take greater effort to regulate.
“Four years after the stunning discovery of warehouse emissions in Georgia, the EPA has failed to propose standards to address this source of uncontrolled emissions,” read a comment letter submitted to the EPA last year and signed by 16 environmental, public health, and labor groups, including Earthjustice and the Union of Concerned Scientists. It is also concerning, they wrote, that most sterilization companies fail to publicly disclose the locations of these warehouses.
“If [warehouses] are significant sources of ethylene oxide, we believe they should be covered by the [EPA’s sterilizer] rule,” Darya Minovi, a senior analyst at the Union of Concerned Scientists, told Grist. “I don’t think that the EPA provided a strong enough rationale for why that wouldn’t be considered.”
The agency is expected to finalize its sterilizer rule in early March. Environmental attorneys said the EPA’s reasoning for overlooking warehouses may come down to legalese. The agency issues regulations based on categories of pollution sources defined in amendments to the Clean Air Act in the 1990s. Since off-site storage facilities weren’t clearly defined in the law decades ago, whether the EPA can regulate them with a rule targeting sterilization facilities is an open question.
A spokesperson for the EPA did not comment on why the agency isn’t including warehouses in its current sterilizer rule but said that it has already determined the levels of ethylene oxide that would be harmful to workers and plans to address emissions in a separate pesticide rule before the end of the year.
Ira Montgomery takes 26 pills a day. There’s one to calm the spasms that ripple through his muscles, another to lower his blood pressure, and yet another to make sure his body doesn’t reject a liver that doctors transplanted after diagnosing him with cancer.
The 51-year-old traces his problems to the years he spent working at the ConMed warehouse. The facility sprawls out over an area the size of five football fields and has rows of shelves that store wooden pallets, each containing medical devices sterilized at a facility owned by Sterigenics about 30 minutes away in Smyrna, Georgia. The products are stored anywhere from a few weeks to several months before they are shipped off to hospitals for use in life-saving procedures.
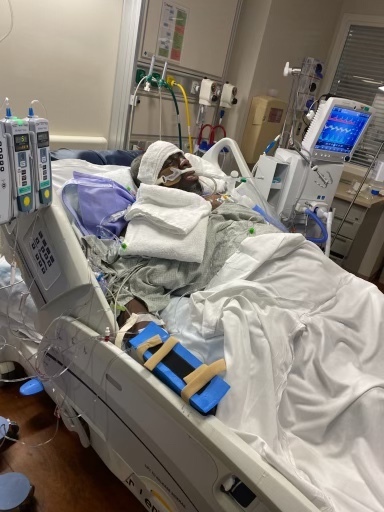
Ira Montgomery lies in the hospital after surgery. He was diagnosed with cancer and needed a liver transplant.
Courtesy of Ira Montgomery
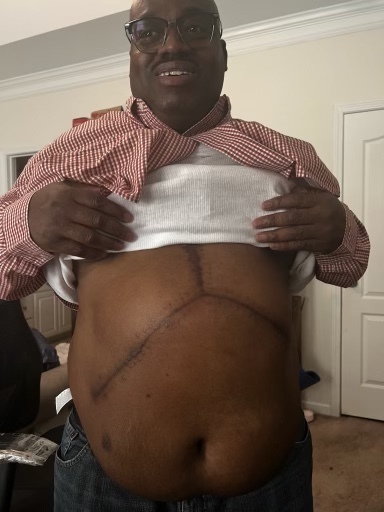
Ira Montgomery shows a scar from his liver transplant. He believes his health problems are linked to his time working at the ConMed warehouse.
Courtesy of Ira Montgomery
In records submitted to the EPA, Sterigenics responded “No” to a question about whether products sterilized at its facilities are shipped to a warehouse, since ConMed contracts with Sterigenics to sterilize products. Given this, the presence of ethylene oxide emissions at the ConMed warehouse only became public knowledge due to Georgia regulators’ efforts. The 7-acre warehousing facility in Lithia Springs had neither state permits nor any protective gear for workers between 2008 and 2019, when Montgomery worked there.
Until an inspection by OSHA in 2019, the 3,500 workers at the warehouse were unaware that the products had been sterilized with a dangerous chemical. Dozens of Montgomery’s and Pittman’s coworkers mysteriously developed rashes, had trouble breathing, and fainted; many had seizures. When Nick Jackson began experiencing seizures in 2007 and eventually died in 2013, ConMed managers told his wife the fluorescent lights at the warehouse were to blame. They later informed his doctors that there were no occupational hazards at his workplace.
Similarly, when Essence Alexander opened a box containing products sterilized with ethylene oxide, her right arm broke out in hives and her body began to itch. Warehouse managers called an ambulance, but when paramedics arrived they were not informed that products at the warehouse were emitting a toxic chemical that could have triggered the reaction. In another case, when a worker broke out in hives all over her body, a manager told paramedics the worker had “an allergic reaction to a cookie,” according to legal filings.

Michael Yeh was working as a medical toxicology fellow at Emory University’s School of Medicine in 2021 when a dozen ConMed workers visited the center. As he got to know these patients better, Yeh began to notice certain patterns in their symptoms. Many felt fine on the weekends, but as soon as the work week rolled around, they developed headaches in the afternoon. Once they got home, the pain would subside. There were other common complaints as well: an irksome cough, itchy eyes, and inflamed nasal passages. These workers weren’t looking for a cure for their conditions, Yeh recalled, but an opportunity to understand how their exposure to ethylene oxide may have affected their health.
“There is no antidote to inhaling ethylene oxide,” Yeh said in an interview. “But they wanted us to hear their story and to document in a medical record what they were experiencing so that there’s documentation of what happened to them.”
ConMed warehouse managers attributed these health effects to a range of unrelated issues, according to a lawsuit filed by about 50 workers in 2020. In filings with the court, the company has argued that the workers did not provide sufficient evidence about their individual exposures and subsequent ailments. Since Pittman, Montgomery, and the other plaintiffs submitted multiple claims to the Georgia Workers’ Compensation Board, ConMed has also claimed that the workers have foregone other avenues to air their grievances. The workers are attempting an “end run around the Workers’ Compensation Act … based on a smattering of vague factual allegations interspersed among a series of legal conclusions,” the company claimed in filings.
In total, the lawsuit counts approximately 50 instances of ambulances being called to the warehouse between 2007 and 2019. A Grist review of 911 call records shows that since 2016, ambulances were dispatched to the ConMed warehouse to treat employees on at least 22 occasions. Multiple workers reported similar health effects: seizures, losing consciousness, vomiting, and trouble breathing. All of these symptoms are triggered by high-dose exposure to ethylene oxide. According to medical management guidelines developed by the Agency for Toxic Substances and Disease Registry, which develops profiles for hazardous substances, ethylene oxide is a central nervous system depressant, and acute exposure to it “can result in diverse neurologic manifestations including seizures, loss of consciousness, and coma.” Exposure to lower concentrations can cause nausea and vomiting, while exposure via skin may cause “inflammation with redness of the skin, blisters, and crusted ulcerations.”

Due in part to these risks, OSHA has set acceptable ethylene oxide exposure limits for workers. The federal agency requires companies to inform workers if they are exposed to more than 0.5 parts per million, or ppm, of ethylene oxide and take measures to reduce exposure when levels are above 1 ppm.
OSHA’s efforts to investigate ConMed’s storage center in Lithia Springs in 2019 point to the warehouse owners’ anxieties over the possibility of being regulated. Grist reviewed OSHA complaint records about the facility and found that federal inspectors’ efforts to sample the indoor air were repeatedly rebuffed.
“The employer was not forthcoming in providing the requested documentation,” the complaint read, adding that two subpoenas had been issued to the company to request information about working conditions, but that the company lawyer rejected both. The initial complaint alleged that airborne ethylene oxide inside the warehouse was causing workers to experience “headaches, burning eyes, itching eyes, cough, and chest pains.” It took five months for regulators to get access to the facility and take air samples and another two months to publish the results.
Those results ultimately indicated that ethylene oxide was present in the air at ConMed, but not at levels that breach federal standards. In handwritten notes attached to the complaint file, an OSHA inspector noted that managers instructed workers not to open packages during the inspection, raising the possibility that managers altered operating procedures on that day. “They didn’t know I can see them,” the inspector wrote.
“Did they change their practices when they knew the inspectors were coming?” Yeh wondered, noting that it would be easy for the company to make adjustments if it knew the inspection date in advance.
The investigation also found that warehouse managers had removed an indoor air quality monitor because it routinely recorded levels between 3 and 5 ppm and beeped loudly. The levels of ethylene oxide were so high, the workers claimed, that the cardboard packages wrapped in plastic shrink-wrap became wet. (Ethylene oxide converts into ethylene cholohydrin and ethylene glycol when it interacts with plastic, and the lawsuit claims that the wet boxes were evidence of the high levels of ethylene oxide that the products were off-gassing.)
OSHA fined ConMed $7,800 for failing to inform workers of their high exposure to ethylene oxide and required the company to install equipment that reduced levels of the chemical. ConMed complied by opening bay doors for better ventilation and installing a stationary air quality monitor, but the monitor continued to detect high ethylene oxide levels.
The workers’ lawsuit claims that the products were off-gassing dangerous levels of ethylene oxide in part because Sterigenics, the sterilization company that shipped products to the ConMed warehouse, was using a sterilization method that requires overapplication of the chemical. Ethylene oxide is an effective disinfectant because even at low levels it can eliminate microbes effectively, but different volumes of the chemical may be required to properly disinfect different products. (The amount of ethylene oxide required to sterilize a product is based on regulations by the Food and Drug Administration.)

Because companies often sterilize different types of products all in one fell swoop, they end up gassing them with more ethylene oxide than is required. The lawsuit claims that Sterigenics used this process — called “overkill” in industry parlance — to sterilize more products quickly. The sterilized products are supposed to be held for several hours to days to allow the ethylene oxide to off-gas before they are sent to warehouses for storage, but the lawsuit claims that Sterigenics rushed this process to increase profits. As a result, the products that were trucked to the warehouse had higher levels of ethylene oxide than anyone would have had reason to suspect at the time.
“The method of sterilization has been to over sterilize,” said Brown, the Earthjustice attorney.
“The result for communities is that they are exposed to higher amounts of ethylene oxide, because more ethylene oxide is being used than necessary.”
Sterigenics did not respond to specific questions about its sterilization methods. In an emailed statement, Kristin Gibbs, a spokesperson for the company, noted that Sterigenics sterilized products provided by ConMed on a contract basis “as required by FDA regulations and standards.”
“The facility in Lithia Springs belongs to ConMed — not Sterigenics — and as such, the facility, the employees working in that facility, and those employees’ working conditions are under the control of and are the responsibility of ConMed,” she added.
In 2019, after OSHA investigated and the EPA identified the Sterigenics facility as a major source of ethylene oxide emissions, workers at the ConMed warehouse began trying to take precautions. Pittman asked her fiancé to buy her a mask, but when she wore it at work, the lawsuit alleges that managers told her not to because she was “scaring” others. Several workers were fired after they began raising concerns about the levels of ethylene oxide at the facility.
Since the EPA hasn’t proposed specific regulations for warehouses, Boylan, the Georgia EPD air chief, said states “should work closely with their warehouses to gather site-specific information on ethylene oxide emissions and try to calculate the risk associated with those emissions.”
“Georgia has done kind of above and beyond, because we thought there were possible risks to nearby communities, and we thought the risks were too great,” said Boylan.
In January, the workers elected to drop ConMed from the lawsuit in part because some of their claims were dismissed in 2022 by the judge presiding over the case. Since the workers had filed claims with the Georgia Workers’ Compensation Board, they were barred from seeking redress through the courts. The judge also dismissed certain claims against Sterigenics, but the company remains a party to the lawsuit. “Sterigenics is vigorously defending the limited surviving claims against it,” said Gibbs, the Sterigenics spokesperson.
Since the lawsuit was filed in 2020, four workers who signed up as plaintiffs have died. Mitchell, Pittman’s fiancé, said that working at the ConMed facility completely changed the trajectory of her life. She was a friendly person, always smiling, but the health complications have dimmed the light in her eyes.
“She was proud of her job and how she had moved up,” said Mitchell. “She always talked about how sad it was and how it hurt her. She was just trying to earn a living and do her job.”
Editor’s note: Earthjustice is an advertiser with Grist. Advertisers have no role in Grist’s editorial decisions.